18 Calculate Energy Of Photon Given Wavelength
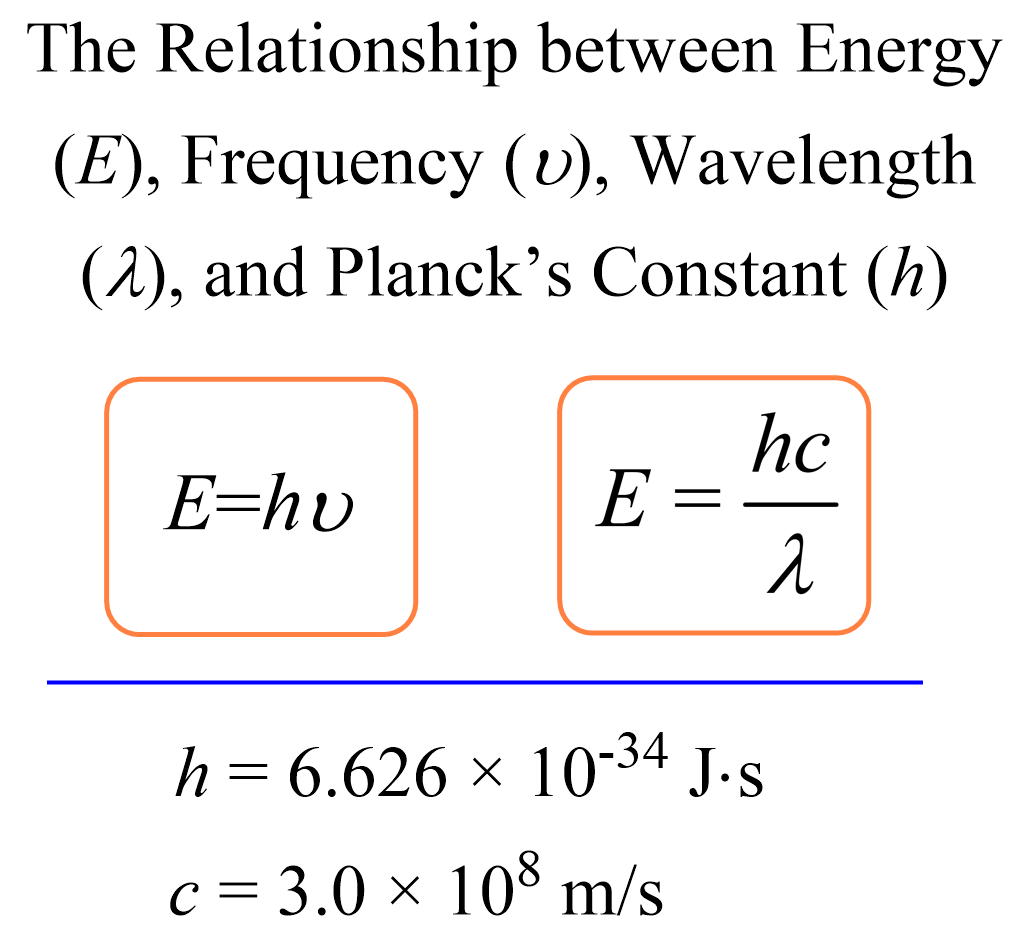
Calculating The Energy Of A Photon Chemistry Steps In order to convert a wavelength to energy in electronvolts (ev): utilize planck's energy equation e = h × c λ. substitute the values of the wavelength (λ), planck's constant (h = 6.6261 × 10⁻³⁴ j⋅s), and speed of light (c = 299792458 m s). you'll get a result in joules (j). to go from joules (j) to electronvolts (ev), use the. F =c λ. where c is the speed of light, f the frequency and λ the wavelength. if you know the frequency, or if you just calculated it, you can find the energy of the photon with planck's formula: e = h × f. where h is the planck's constant: h = 6.62607015e 34 m² · kg s. 3. remember to be consistent with the units!.

How To Calculate The Energy Of A Photon Physics Study To calculate photon energy from wavelength: make sure your wavelength is in meters. divide the speed of light, approximately 300,000,000 m s, by the wavelength to get the wave's frequency. multiply the frequency by planck's constant, 6.626×10 −34 j hz. the resulting number is the energy of a photon!. Use the equation above and input the wavelength of the photon's electromagnetic radiation. the result will be the energy the photon is carrying. you can also easily switch from nm to ev with this wavelength to energy calculator. simply select these units in the dropdown list next to each field. if you want to convert energy to wavelength. An online photon energy calculator that allows you to calculate the energy of a photon from its wavelength (ƛ) & frequency (f). in simple words, with the ease of this online tool, you can explore the relationship between the frequency, wavelength and the energy. Example formula. the energy of a photon can be calculated using the following formula: e = hc λ. where: e is the energy of the photon (in joules). h is the planck 's constant (6.62607015 × 10 34 m 2 kg s). c is the speed of light (approximately 3 × 10 8 m s). λ is the wavelength of the photon (in meters).
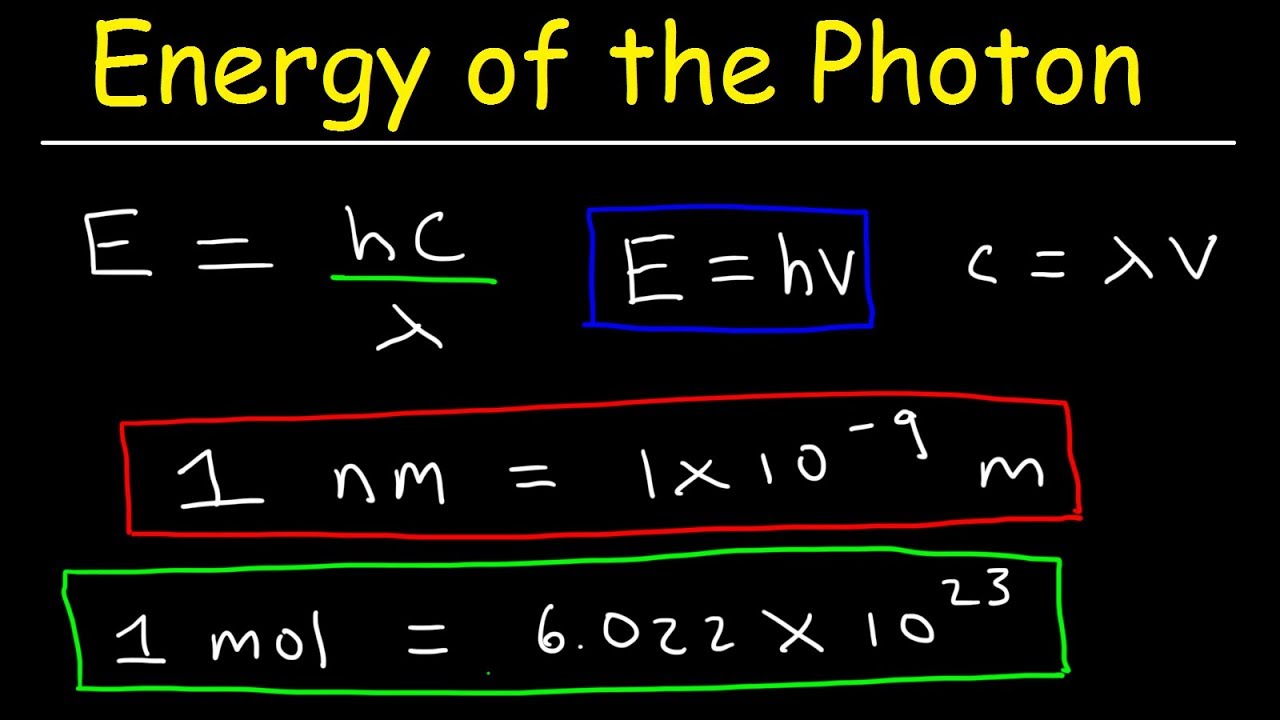
How To Calculate The Energy Of A Photon Given Frequency Wavelength In An online photon energy calculator that allows you to calculate the energy of a photon from its wavelength (ƛ) & frequency (f). in simple words, with the ease of this online tool, you can explore the relationship between the frequency, wavelength and the energy. Example formula. the energy of a photon can be calculated using the following formula: e = hc λ. where: e is the energy of the photon (in joules). h is the planck 's constant (6.62607015 × 10 34 m 2 kg s). c is the speed of light (approximately 3 × 10 8 m s). λ is the wavelength of the photon (in meters). This calculator computes the energy of a photon from its vacuum wavelength \lambda λ, frequency \nu ν or wavenumber \kappa κ. the photon energy is. where h \approx 6.626\cdot 10^ { 34} h ≈ 6.626 ⋅10−34 is the planck constant and c c is the speed of light in vacuum. energy is converted to electronvolts by dividing it by the elemental. Since light is quantized, we can calculate the number of photons in a light beam of given energy and frequency by simply dividing the energy of the beam by the result of the formula for the energy of a photon: n {\text {ph}}=\frac {e {\text {beam}}} {h\cdot \nu} nph = h ⋅ ν e beam. davide borchia. with the photon energy calculator you will.

Photon And Energy Levels This calculator computes the energy of a photon from its vacuum wavelength \lambda λ, frequency \nu ν or wavenumber \kappa κ. the photon energy is. where h \approx 6.626\cdot 10^ { 34} h ≈ 6.626 ⋅10−34 is the planck constant and c c is the speed of light in vacuum. energy is converted to electronvolts by dividing it by the elemental. Since light is quantized, we can calculate the number of photons in a light beam of given energy and frequency by simply dividing the energy of the beam by the result of the formula for the energy of a photon: n {\text {ph}}=\frac {e {\text {beam}}} {h\cdot \nu} nph = h ⋅ ν e beam. davide borchia. with the photon energy calculator you will.

Wavelength Energy Equation

Comments are closed.